01
Before
Raziel Completed 10 Successful Clinical Studies in the U.S. for Various Indications

Submental Fat – Phase 2b
Flanks – Phase 2a
Dercum – Phase 2b
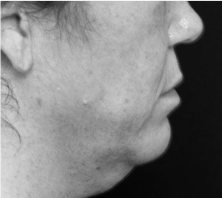
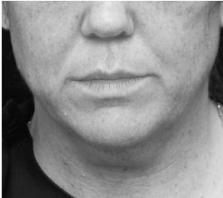
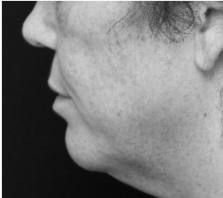
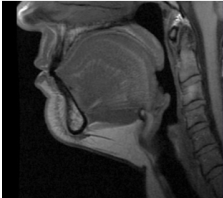
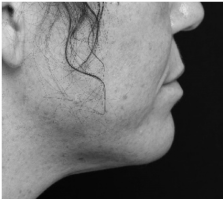
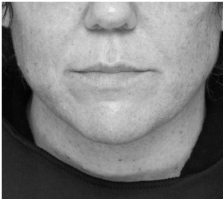
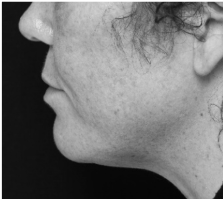
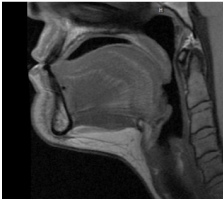
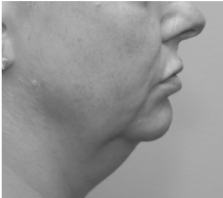
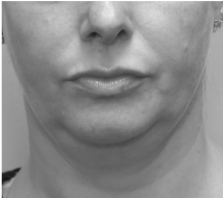
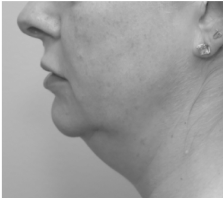
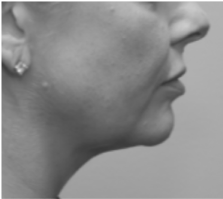
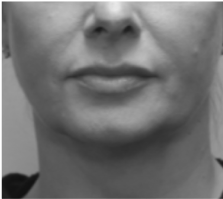
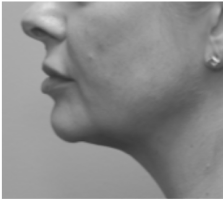
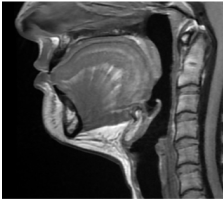
Submental fat, or “double chin”, is a layer of fat beneath the chin. Many individuals pursue treatments to reduce submental fat, enhancing their profile and rejuvenating the neck area.
In a double-blind, placebo-controlled study, conducted across 12 sites in the USA (n=151), single injection session of RZL-012 (240+/-30mg) showcased both safety and effectiveness for submental fat reduction.
01
Before
01
After
02
Before
02
After
Body contouring is a sought-after aesthetic procedure that aims to reshape body areas that contain excessive fat. It’s a process that can transform one’s appearance, creating a more sculpted and harmonious silhouette. Raziel’s first indication in this field targets the flanks, areas of fat on the sides of the body near the waist that can disrupt the body’s natural flow.
A double-blind, placebo-controlled study was conducted to evaluate the safety and efficacy of RZL-012 in subjects seeking focal flank contouring. The study involved 12 subjects, in which one flank was treated with RZL-012 (412 mg) and the other with a matching placebo through a single injection cycle. The study showed initial promising results in fat reduction and safety.
Before
Left flank injected with placebo, Right Flank injected with RZL-012
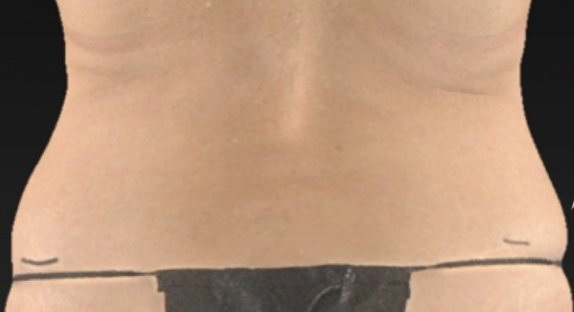
Screening 3D
After
Left flank injected with placebo, Right Flank injected with RZL-012
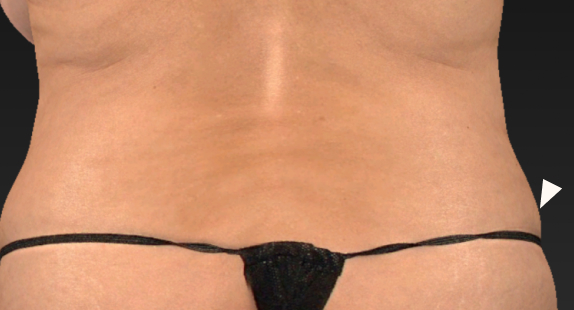
Week 12 3D
Dercum’s disease is a rare disorder characterized by painful lipomas that form beneath the skin.
In a double-blind study, a total of 38 subjects were randomized to either RZL-012 or Placebo, treating an average of 6 lipomas per subject. The study found RZL-012 to be more effective in pain relief than the placebo, supporting the potential for a phase 3 study focusing on lipoma pain reduction.
Secondary end point: Reduction in Pain (%), 84 days after treatment
Lipoma pain was significantly reduced after RZL vs. Placebo treatment

Office:
+972-8-9126940Email:
[email protected]Location:
10 Prof. Menachem Plaut St.,
Rehovot, Israel